Container Closure and Integrity Testing
Coriolis Pharma offers industry-leading container closure integrity (CCI) testing services to ensure the sterility and stability of your drug products throughout their shelf life. Utilizing our scientific expertise and state-of-the-art technologies, we provide comprehensive CCI testing solutions that support your regulatory compliance and product safety requirements.
Container Closure Selection and Integrity Testing Tailored to Your Needs
Coriolis offers comprehensive testing of the container closure and the integrity of primary packaging materials, including rubber-stoppered glass vials, prefilled syringes and double-chamber cartridges. We also screen different primary packaging formats and products to identify the most suitable option for your drug product and production process.

Glass Vials and Rubber Stoppers
Glass vials are commonly used as a versatile primary container for biopharmaceuticals. Both liquid and lyophilized formulations can be stored, whereas the latter are also freeze-dried directly inside the vial. We utilize a range of analytical techniques, many of them noninvasive, to verify that the oxygen and moisture level inside the headspace of the vial remains unchanged over the intended shelf life and even under accelerated stress conditions, following ICH guidelines or custom conditions.
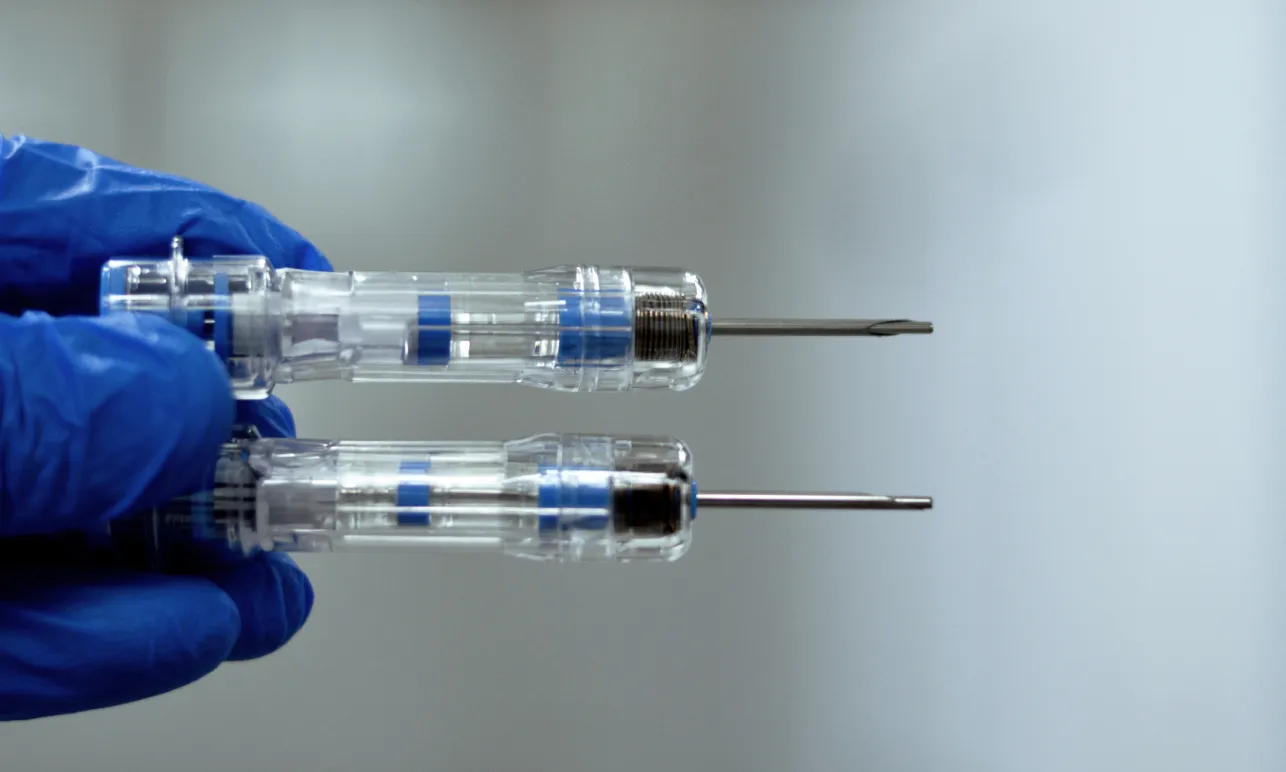
Evaluation of Prefilled Syringes
Prefilled syringes are often used to enable patient self-administration. Elderly people, rheumatoid patients or children need to be able to use the syringe by themselves but may not be able to apply significant force on the plunger. We test the break-loose and gliding forces of prefilled syringes and double-chamber cartridges and compare different syringe and needle combinations to find the most suitable option for your drug product.
Container Closure, Container Integrity and Primary Packaging Testing Methods
We can select the most suitable approach for your analytical challenge, whether following a standardized in-house workflow or a custom-tailored solution.
Analytical Method Development, Qualification and Validation
For common sample types, we can often apply standardized methods with little setup effort. However, when needed, our experienced analytical experts create or optimize custom methods tailored to your active pharmaceutical ingredient, product type and development phase.
Container Closure and Integrity for Your Drug Product
Coriolis Pharma’s deep expertise across many biologic modalities, combined with input from world-leading external advisors, offers the knowledge and experience needed to solve even the most complex formulation and drug development challenges.
Your Phase
Our expert team has the expertise and experience to guide your biopharmaceutical development program from preclinical and early-phase development to the market.
Container Closure Integrity Testing Resources

Publications
March 27, 2025

Press Releases
March 11, 2025

Publications
October 8, 2024
